
Program Management & Risk Management
We unite and organize the many partners needed to deliver major programs.
Complex projects require hundreds if not thousands of players. From managing each line of the project schedule, to uniting and organizing disparate agents, we have the reach to make sure that every entity involved has complete clarity on what they are meant to do, and when.
Our breadth of expertise means that we understand how to distribute roles across a range of trades and techniques efficiently and effectively. Importantly, we then remain a consistent presence throughout the project duration, providing a crucially common point of reference as partners and suppliers share and cooperate on often enormous scales.
Building a strong foundation for product approval and commercial success
The FDA may require a Risk Evaluation and Mitigation Strategy (REMS) so it can approve or continue to approve a prescription drug to ensure the benefits outweigh the risks. In some cases, the FDA may require brand and generic sponsors to jointly develop and implement a REMS (called a shared system REMS).
When this happens, it raises an always-complex challenge: How to enable successful collaboration among marketplace competitors in the design, development and implementation of single shared programs that cover multiple assets?
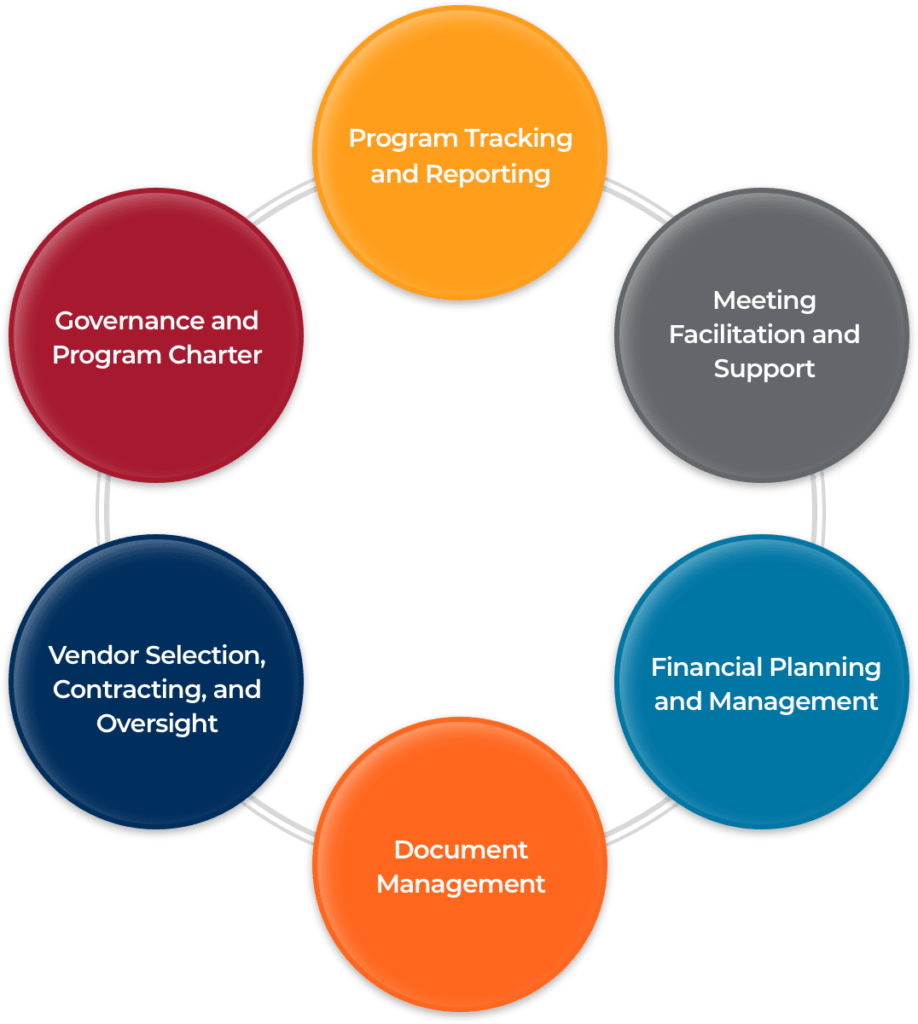
In these cases, it is crucial to have the support of an experienced Program Management Office (PMO).
Our Risk & Program Management team can be your PMO, or we can support individual service needs for many postmarketing requirements/commitments
Project management processes and methods to help you manage complexity for all of your post-market regulatory needs
Our Risk & Program Management Advisory Group supports pharma clients through the development, implementation, and ongoing management of single and shared Risk Evaluation & Mitigation Strategies (REMS) and other post-marketing risk minimization projects Services include:
- Supplemental staff for clients needing associates with REMS and/or post-marketing risk minimization knowledge
- Project management support and expertise
- REMS and post-marketing risk minimization strategy and regulatory consulting
- Submission and assessment support
- Grant management system and support (e.g., continuing education, educational grants, investigator-sponsored research)
- Type V drug master file (DMF) holder and agent
- Knowledge, attitude, and behavior (KAB) surveys
- Usability testing, focus groups, and market research
- Food & Drug Administration (FDA) advisory committee meeting support
- PMO support for multi-sponsor and/or multi-stakeholder programs (e.g., shared REMS, post-marketing requirements/commitments, global risk management plans)
- Vendor procurement and management